GERMINATION.
Not
observed.
MYCORRHIZAE.
The sporocarps of Gl. badium found by the author of this website were formed among and occasionally on vesicular-arbuscular mycorrhizal roots of Acer palamatum Thunb., Agropyron repens (L.) P. Beauv., Agrostis stolonifera L., Allium schoenoprasum L., Ammophila arenaria (L.) Link, Anthriscus sylvestris (L.) Hoffm., Avena sativa L., Cirsium arvense (L.) Scop., Corynephorus canescens (L.) P. Beauv., Crataegus monogyna Jacq., Equisetum arvense L., Festuca arundinaceae Schreb., Festuca ovina L., Ficaria verna Huds., Glycine max (L.) Merr., Helictotrichon pubescens (Huds.) Pilg., Heracleum sphondylium L., Hordeum vulgare L., Hypericum perforatum L., Juncus effusus L., Juniperus communis L., Lupinus luteus L., Malus x purpurea Rehder, Rosa rugosa Thunb., Rubus idaeus L., Rumex acetosella L., Silene latifolia Poir. ssp. alba (Mill.) Greuter et Burdet, Thuja occidentalis L., Trifolium pratense L., Triticum aestivum L., and Zea mays L. In trap cultures with root and rhizosphere soil mixtures of the plant species sampled, Gl. badium rarely produced spores. All one-species cultures of this fungus established were unsuccessful. Oehl et al. (2005) also failed to obtain Gl. badium in one-species cultures and, hence, properties of mycorrhizae of this fungus remain unknown. However, phylogenetic molecular analyses showed Gl. badium to be a member of Glomus Group A (Oehl et al. 2005) sensu Schwarzott et al. (2001), comprising fungi known to form vesicular-arbuscular mycorrhizae (Błaszkowski 2003).
PHYLOGENETIC
POSITION. Phylogenetic molecular analyses showed Gl. badium to be a member of Glomus Group A (Oehl et al. 2005) sensu Schwarzott et al. (2001).
DISTRIBUTION. In Poland, Gl. badium was recovered from 39 field-collected mixtures of roots and rhizosphere soils. Of them, 15 came from under nine species of cultivated plants, and the others represented 20 species of wild plants.
The spore abundance of Gl. badium was much higher among roots of wild plants (av. 126.3; range 1-850 in 100 g dry soil) than cultivated plants (av. 5.2; range 1-20 in 100 g dry soil). The proportion of spores of this fungus in spore populations of all arbuscular fungi isolated also was higher in the root zone of wild plants (av. 45.2%; range 0.9-100%) than cultivated ones (av. 10.8%; range 0.9-40.7%). The species abundance in the root and soil samples taken from under cultivated plants averaged 5.0 with a range of 4-7 in 100 g dry soil, and that under wild plants averaged 3.7 and ranged from 1 to 9 in 100 g dry soil.
The arbuscular fungi co-occurring with Gl. badium were Acaulospora polonica Blaszk., A. capsicula Blaszk., A. cavernata Blaszk., A. gedanensis Blaszk, A. lacunosa J.B. Morton, A. mellea Spain & N.C. Schenck, A. paulinae Blaszk., an unrecognized Acaulospora sp., Gl. aggregatum N.C. Schenck & S.M. Sm. emend. Koske, Gl. caledonium (Nicol. & Gerd.) Trappe & Gerd., Gl. constrictum Trappe, Gl. deserticola Trappe, Bloss & J.A. Menge, Gl. ? etunicatum W.N. Becker & Gerd., Gl. fasciculatum (Thaxt.) Gerd. & Trappe emend. C. Walker & Koske, Gl. fuegianum (Speg.) Trappe & Gerd., Gl. geosporum (Nicol. & Gerd.) C. Walker, Gl. macrocarpum Tul. & C. Tul., Gl. microcarpum Tul. & C. Tul., Gl. mosseae (Nicol. & Gerd.) Gerd. & Trappe, unrecognized Glomus spp., Paraglomus occultum (C. Walker) J.B. Morton & D. Redecker, Scutellospora armeniaca Blaszk., and Scutellospora dipurpurescens J.B. Morton & Koske.
Apart from Poland, Gl. badium has been found among roots of grasses and grassland plants growing in soils of pH 6-8 and located in Germany, France, Switzerland, and Italy (Oehl et al. 2005).
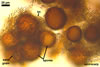
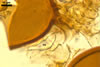
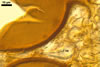
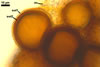
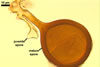
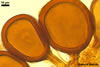
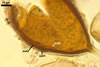
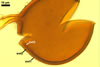
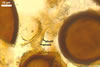
NOTES. The most distinguishing characters of Gl. badium are its small sporocarps lacking a peridium and composed of many, brownish orange to reddish brown, relatively small spores. The innermost flexible to semi-flexible and coloured layer of the three-layered spore wall also is a diagnostic property of this species.
Glomus badium probably forms spores only in multispored sporocarps, and single spores occasionally recovered from field-collected soil samples represented their fragments. Examination of the ontogenesis of this species is needed to confirm this supposition. Unfortunately, all attempts to produce one-species cultures of Gl. badium made by the author of this website and by Oehl et al. (2005) failed.
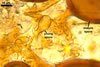
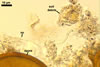
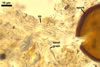
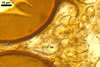
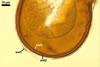
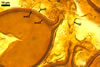
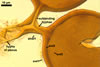
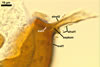
The interspore mycelium of the specimens of Gl. badium found by the author of this website morphologically corresponds to that characterized by Oehl et al. (2005). However, the original description of this fungus does not mention the cystidium-like structures occurring between spores of the Polish sporocarps of this fungus. The function of the cystidia-like structures in sporocarps of Gl. badium probably is similar to that of cystida of Agaricales, in which they appear to serve as spacers between basidia (Ulloa and Hanlin 2000).
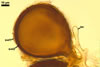
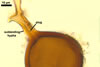
Of the three wall layers of spores of Gl. badium, the two outer ones are typical of most species of the genus Glomus, in which the outermost layer sloughs with age and adheres to a laminate structural layer. The third spore wall layer of Gl. badium is thin, pale yellow (4A3) to light brown (6D8), and usually tightly adheres to the inner surface of the brownish orange (6C8) to reddish brown (8E8) layer 2 in even vigorously crushed spores. Hence, it is difficult to see, especially in young and freshly matured spores. In older spores, this layer usually is slightly thicker and more frequently separates from the middle laminate layer. Additionally, this layer is most visible at the spore base where it forms a straight or curved septum of the subtending hypha continuous with its part adherent to the laminate spore wall layer 2.
Apart from Gl. badium, species of the genus Glomus forming small and compact sporocarps with small, brownish orange to reddish brown spores are Gl. cuneatum McGee & Cooper, Gl. fuegianum (Speg.) Trappe & Gerd., Gl. invermaium I.R. Hall, and Gl. rubiforme (Gerd. & Trappe) R.T. Almeida & N.C. Schenck. Compared with Gl. badium, sporocarps of Gl. cuneatum are much larger (2-12 mm vs. 200-500 x 290-680 µm in Gl. badium) and have a peridium (vs. no peridium in Gl. badium; McGee and Trappe 2002). Additionally, the spore wall of the latter species consists of only one laminate layer lightening from black to hyaline towards their inside, whereas the spore wall of the former fungus comprises three layers, of which the laminate layer is dark- and uniform-coloured.
According to Thaxter (1922), spores of Gl. fuegianum coming from Spegazzini's original collection were reddish brown as are those of Gl. badium. In contrast, all spores of Gl. fuegianum found by the author of this website in Poland, those loaned from Kew, United Kingdom (Błaszkowski et al. 1998), and those revealed in Australia by McGee and Trappe (2002) were pale yellow to yellow brown. Moreover, the spores were frequently surrounded by branched and convoluted hyphae (Błaszkowski 2003) that never occur on spores of Gl. badium (Błaszkowski, pers. observ.; Oehl et al. 2005). Finally, the radial arrangement of spores in sporocarps of Gl. fuegianum is regular, and irregular in sporocarps of Gl. badium.
Glomus badium differs from Gl. invermaium in the formation of smaller (200-500 x 290-680 µm vs. up to 1 mm across) and more compact sporocarps with regularly distributed spores (vs. loose sporocarps with randomly distributed spores in Gl. invermaium; Błaszkowski, pers. observ.; Hall 1977). Other differences are the number and the phenotypic properties of wall layers of spores of these fungi. The spore wall of Gl. badium comprises three layers, of which the outermost one sloughs with age. In contrast, the outermost wall layer of spores of Gl. invermaium is persistent and the spore wall of this species lacks the third spore wall layer of Gl. badium.
The main difference between Gl. badium and Gl. rubiforme resides in the wall structure of their spores. The spore wall of Gl. rubiforme comprises only two layers similar in colour and phenotypic properties to layers 1 and 2 of the spore wall of Gl. badium (Błaszkowski 2003; Gerdemann and Trappe 1974).
REFERENCES
Błaszkowski J. 2003. Arbuscular mycorrhizal fungi (Glomeromycota), Endogone and Complexipes spec ies deposited in the Department of Plant Pathology, University of Agriculture in Szczecin, Poland. Address: http://www.agro.ar.szczecin.pl/~jblaszkowski/.
Błaszkowski J., Madej T., Tadych M. 1998. Entrophospora baltica sp. nov. and Glomus fuegianum, two species in the Glomales from Poland . Mycotaxon 68, 165-184.
Gerdemann J. W., Trappe J. M. 1974. The Endogonaceae in the Pacific Northwest. Mycological Memoir 5, 1-76.
Hall I. R. 1977. Species and mycorrhizal infections of New Zealand Endogonaceae. Trans. Brit. Mycol. Soc. 68, 341-356.
McGee P. A., Trappe J. M. 2002. The Australian zygomycetous mycorrhizal fungi. II. Further Australian sporocarpic Glomaceae. Austral. Syst. Bot. 15, 115-124.
Oehl F., Redecker D., Sieverding E. 2005. Glomus badium, a new sporocarpic mycorrhizal fungal species from European grasslands with higher soil pH. J. Appl. Bot. and Food Qual. 79, 38-43.
Schwarzott D., Walker C., Schuessler A. 2001. Glomus, the largest genus of the arbuscular mycorrhizal fungi (Glomales) is nonmonophyletic. Mol. Phylogen. Evol. 21, 190-197.
Thaxter R. 1922. A revision of the Endogonaceae. Proc. Am. Acad. Arts and Sci. 57, 291-351.
Ulloa M., Hanlin R. T. 2000. Illustrated Dictionary of Mycology. APS Press. The American Phytopathological Society. St. Paul, Minnesota.